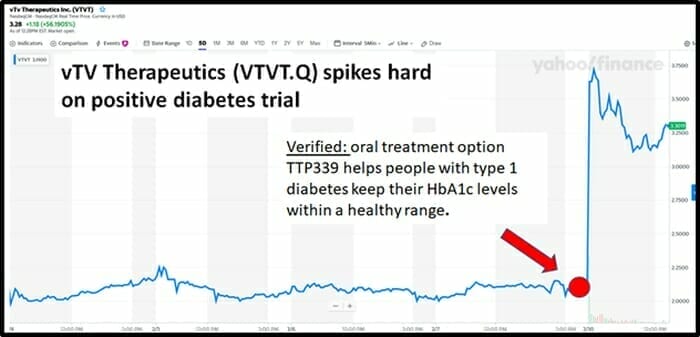
On February 10, 2020 vTv Therapeutics (VTVT.Q) gained $100 million in market cap (surging from $2.08 to $3.24) after the publication of results from a trial assessing TTP399 as an oral adjunctive therapy to insulin in adults with type 1 diabetes (T1D).
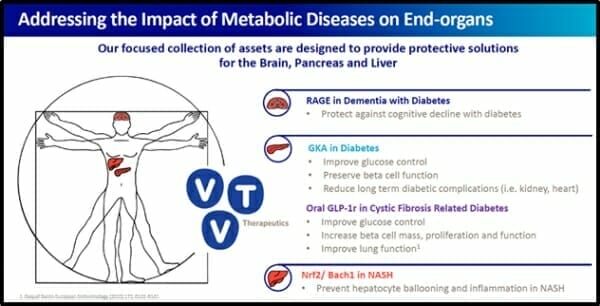
vTv Therapeutics is a clinical-stage pharmaceutical company focused on treating metabolic diseases through end-organ protection. It does this by advancing clinical drug candidates into safe and effective medicines.
According to the IDF Diabetes Atlas, “every seven seconds someone is estimated to die from diabetes or its complications, with 50% of those deaths (4 million per year) occurring under the age of 60 years.”
“Recently I was told that I have diabetes type 1,” blogged Denmark university student Julie, “I still struggle with the thought ‘I have diabetes’, just typing it makes me feel a little uncomfortable.”
“My GP said that I have depression tendencies, probably due to my illness,” continued Julie, “I live with my roommate in the student dorm. I almost ended up in a coma, as my friends did not know what to do. I made the mistake of taking my insulin but forgot to eat.”
vTv Therapeutics’ study was conducted with support from JDRF International (JDRF).
JDRF is a non-profit that funds T1D research and advocates for regulation favorable to medical research. In 2018, JDRF provided $85 million (37% of their total income) to T1D scientific research grants.
“The results from the Simplici-T1 trial indicate that TTP339 is a promising oral treatment option to help people with type 1 diabetes keep their HbA1c levels within a healthy range,” stated Dr. Sanjoy Dutta, JDRF V.P of Research, “while simplifying the daily management of the disease.”
Keeping HbA1c levels in a healthy range is a big deal for people with type 1 diabetes.
Biotech investors often use the term HbA1c levels synonymously with blood glucose levels but they are not the same thing.
A blood glucose level is a concentration of glucose in your blood at a single point in time (at the moment of the test).
HbA1c test serves as an overall marker of what your average levels are over a period of 2-3 months.
How does HBA1c perform this backward-facing diagnostic trick?
Glucose in the bloodstream attaches to haemoglobin. The amount of glucose that combines with this protein is a function of the total amount of sugar that is in your system at that time.
Because human red blood cells survive for 8-12 weeks before renewal, measuring HbA1c gives you a snapshot of the average blood glucose levels over the last 2-3 months.
The higher the HbA1c, the greater the risk of developing diabetes-related complications.
A UK Prospective Diabetes Study (UKPDS) demonstrated that “improving HbA1c by 1% for people with type 1 diabetes or type 2 diabetes cuts the risk of microvascular complications by 25%.”
If that’s all too geeky, you can watch this explainer video:
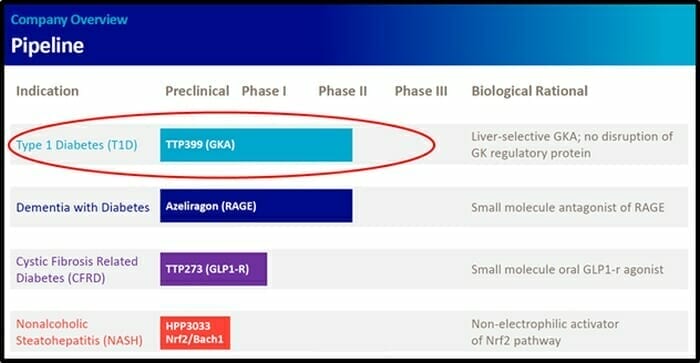
vTv Therapeutics’ TTP399 is a liver-selective glucokinase activator taken once a day.
The 12-week trial investigated the efficacy and safety of 800 mg of TTP399 compared with placebo in 85 people with type 1 diabetes on optimized insulin therapy.
TTP399, a novel glucokinase activator, achieves primary objective of a statistically significant reduction in HbA1c, without increases in hypoglycemia or ketoacidosis.
The trial achieved its primary objective by demonstrating statistically significant improvements in HbA1c (long-term blood sugar) for TTP399 compared to placebo at week 12.
“A once-a-day pill that reduces HbA1c and improves time in range with continuous glucose monitoring, without increasing hypoglycemia or any signal for adverse events, is a big win for the future care of type 1 diabetes,” stated clinical researcher Dr. John Buse.
“Despite advances in insulin and its https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration, people with T1D continue to have difficulty achieving optimal glucose control (HbA1c of less than 7.0%), warranting the need for adjunctive therapies,” declared vTv Therapeutics.
“Roughly 1.5 million people in the US are living with type 1 diabetes and the burdensome, around the clock disease management it requires to avoid life-threatening complications,” stated Steve Holcombe, President and CEO of vTv Therapeutics, “These patients and their families are demanding new treatment.
vTv Therapeutics has a pipeline of small molecule clinical and pre-clinical drug candidates for the treatment of metabolic diseases such as type 1 diabetes and Alzheimer’s disease.
“We intend to engage with the FDA as soon as possible to plan an efficient development pathway for TTP399 and hope to initiate a registration trial this year,” stated vTv Therapeutics.
Clinical trial registration is the practice of documenting clinical trials before they are performed, to combat “publication bias” and “selective reporting”.
The registration of a clinical trial makes it impossible to bury negative results, forcing the company tell the public and the scientific community what they’ve learned from the trial – whether it benefits them or not.
Globally, registration of clinical trials is being standardized. Some top medical journals will only publish the results of trials that have been pre-registered.
Big Pharma companies like GlaxoSmithKline (GSK.NYSE), Merck (MRK.NYSE) and Pfizer (PFE.NYSE) are investing heavily in diabetes treatment.
We recently wrote about three microcap Canadian companies Resverlogix (RVX.T), Sirona Biochem (SBM.V), Sernova (SVA.V) – that are also operating in the same space.
On February 10, 2020 by mid-morning, vTv Therapeutics share price was up 53% to $3.23 on 19 million shares traded.
– Lukas Kane

Leave a Reply