Three times a month, my sister-in-law is viciously attacked without warning.
In the wake of these attacks, the pain is so great that she can’t speak.
She hides under the covers of her bed, afraid to move.
The symptoms last between 3 hours and 48 hours.
If she tries to walk, she falls down.
She describes the pain as “like suffering from food poisoning while being hit in the head with a baseball bat”.
At her bedside, she has a potpourri of medications. They don’t always work. Some of them induce vomiting – which greatly increases the pain.
Her attacker?
Migraine headaches.
“More than 4 million adults experience chronic daily migraine – with at least 15 migraine days per month,” states The Migraine Research Foundation, “It affects 39 million people in the U.S. and 1 billion worldwide.”
The medical cost of treating chronic migraine is more than $5 billion, however, these sufferers spent $41 billion on treating their entire range of conditions.
85% of chronic migraine sufferers are women.

“Expectations were high for three new migraine drugs hitting the market from Amgen (AMGN.Q), Eli Lilly (LLY.NYSE) and Teva Pharmaceuticals (TEVA.NYSE),” reported Reuters a year ago, “Priced around $7,000 each, the drug-makers called them ‘breakthrough’ treatments designed to prevent migraines when taken year-round.”
But a small group of external medical experts who quietly advise U.S. health insurers on new drugs was not impressed. They concluded that all three medicines offered no clear benefit over drugs already on the market and that insurers could consider them optional when it came to health coverage.
Here are three biotech companies addressing the need for more effective migraine medication.
On March 23, 2020 the $2 billion company Biohaven Pharmaceutical (BHVN.NYSE) announced a successful end of Phase 2 clinical and nonclinical interaction with the FDA for intranasal vazegepant for the acute treatment of migraine.
Biohaven is developing therapies to help patients with debilitating neurological and neuropsychiatric diseases.
Intranasal https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration (into the nostrils) is a non-invasive technique for delivering medication.
The surface of the nasal mucosa in humans is 150 cm2, it is well supplied by blood vessels, ensures a rapid absorption of most drugs, while avoiding the “first pass metabolism” following oral https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration.
Intranasal https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration is often used for pain medications, rescue medications or for seizures in children. It is considered ideal for self-https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration or domestic care-givers.
Biohaven reported that intranasal vazegepant achieved statistical superiority to placebo on the co-primary regulatory endpoints of pain freedom and freedom from most bothersome symptoms at 2 hours in a Phase 2/3 dose finding trial.
The company addressed all issues raised by the FDA and will advance the 10 mg dose of intranasal vazegepant into a double-blind, placebo-controlled Phase 3 clinical trial.
“If approved, this novel intranasal formulation of vazegepant will complement our recently approved, NURTEC ODT orally disintegrating tablet, currently marketed for acute treatment of migraine,” stated Vlad Coric, CEO of Biohaven.
Vazegepant is the newest member of Biohaven’s migraine platform to have demonstrated efficacy in a pivotal trial.
“In the prior clinical trial, intranasal vazegepant showed evidence of rapid onset with pain relief as early as 15 minutes,” stated Robert Croop, Biohaven’s Chief Development Officer, “return to normal function at 30 minutes and sustained benefits through 48 hours. Patients with migraine deserve multiple therapeutic options to better treat their migraines and return to their daily lives.”
Biohaven is repped by Sam Brown – a full-service, independent healthcare communications agency helmed by Laura (Mastrangelo) Liotta.
Deploying an army of technical communication wizards, Sam Brown provides PR services to biotechnology, device and pharmaceutical companies in all phases of development and commercialization.
If the planned Phase 3 trial is positive, BHVN could file a New Drug Application (NDA) in 2021.

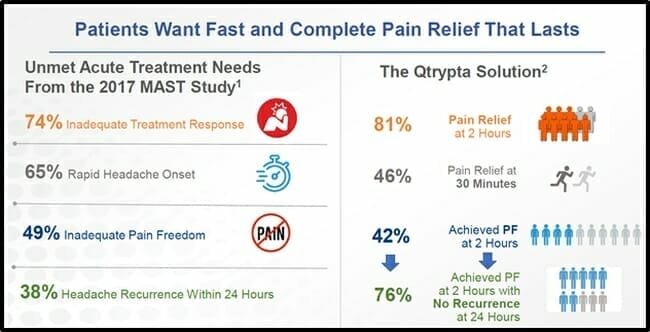
On March 04, 2020 the $30 million company Zosano Pharma (ZSAN.Q), announced that the FDA has accepted the company’s New Drug Application (NDA) for Qtrypta for filing and substantive review.
Zosano Pharma is a clinical-stage biopharmaceutical company developing products where the rapid https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration of approved molecules can provide medical solutions in markets underserved by existing therapies.
Qtrypta uses an intracutaneous microneedle system, consisting of titanium microneedles coated with the drug. The microneedles are designed to penetrate the epidermis and dermis, quickly entering the bloodstream.
“Migraine can significantly impair functional ability at work or school with devastating impact to the patient’s quality of life,” stated Alan M. Rapoport, M.D., a Clinical Professor of Neurology at UCLA, “I believe Qtrypta, if approved, will offer patients with difficult to treat migraine attacks a new, non-oral treatment option that gives them confidence and control over their attacks.”
“The FDA’s filing of our NDA represents a significant milestone for Zosano,” stated Steven Lo, President and CEO of Zosano. “This submission also represents the first NDA to be submitted to the FDA for a pharmaceutical microneedle patch.”
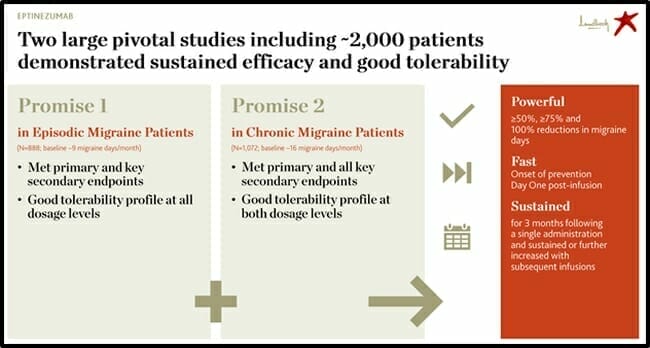
The $5.6 billion company Lundbeck (HLUYY.OTC) recently acquired Alder BioPharmaceuticals, – a biotech focussed on migraine treatment and prevention – in a transaction valued at USD $1.95 billion.
The transaction is expected to amplify and diversify Lundbeck’s revenue growth, through the anticipated (2020) U.S. launch of eptinezumab – a preventive treatment of episodic and chronic migraine.
On February 22, 2020 Lundbeck announced that Vyepti (eptinezumab-jjmr) has been approved by the FDA for the preventive treatment of migraine in adults and will be available in April 2020.
Vyepti is the first FDA-approved intravenous (IV) treatment for migraine prevention.
“With the approval of Vyepti, we are now able to offer a new IV therapy that achieves the key treatment goal of preventing migraine over time while also delivering on the need for earlier onset of efficacy,” stated Deborah Dunsire, Lundbeck President & CEO, “The Vyepti clinical program is the first to demonstrate this early benefit.”
“Eighty-five percent of the market for prevention treatments are older therapies that either have a poor evidence base or are not very effective or have significant tolerability issues,” explained Peter Anastasiou, Lundbeck’s EVP of North America on a recent earnings call, “About 25% of the migraine prevention market are people who are diagnosed but are not currently treated because they have been dissatisfied with the efficacy or the tolerability issues with those older therapies”.
“Women are more than twice as likely to report migraine (20% vs 9%),” states The American Journal of Managed Care, “Numerous studies suggest that women may be more susceptible to migraine due to the way that fluctuations in estrogen levels affect cells in the brain.”
Every year in the U.S, there are 1.2 million visits to the ER for acute migraine attacks.
I just called my sister-in-law to ask her to vet this article for inaccuracies, omissions or distortions.
She didn’t answer her phone.
Hopefully, she’s unplugged.
If not, it’s quite likely she’s drowning in an ocean of pain, praying for a nimble biotech company to toss her a life-buoy.
– Lukas Kane


Leave a Reply