Last week we talked about shit.
Today, we are going to talk about spit.
Specifically, how the properties of spit are playing a crucial role in the global containment of COVID-19.
17 million people worldwide have now contracted the coronavirus, with 670,000 fatalities.
Globally, more than 80 million people have been tested.
Most of that testing is done by inserting a Q-tip deep into a cranial orifice and rotating the Q-tip – in order to collect a mucous sample, using a “nasopharyngeal swab technique”.
“Some patients return multiple negative tests before returning a positive,” states one ICU doctor, “Not getting deep enough for the swab to work is a big part of this”.
Here’s a demonstration of the technique [warning: not suitable for squeamish viewers]:
“It did hurt (briefly, like an injection)” reports one test subject on a Reddit forum, “But it’s not nearly as unpleasant as a pap test.”
“The swab was rotated in each nostril for 15 seconds,” recalled another Reddit user, “It felt like I was drowning.”
No-one is going to die from the pain caused by a “nasopharyngeal swab” – but it is likely that this invasive procedure forms a disincentive to getting tested.
How I wish a clever bio-tech company would develop a reliable COVID-19 screening mechanism that only needed to collect spit – rather than mucus.

On July 6, 2020 XPhyto Therapeutics (XPHY.C) announced that its diagnostic partner, 3a-Diagnostics is developing an affordable, portable screening tool that only requires the collection of saliva.
That means you don’t need a Registered Nurse to penetrate your skull with a Q-tip – to a get a sample.
“We believe that a low-cost, portable and easy to use screening tool that provides rapid on-the-spot results would be a disruptive tool in the fight against pandemic threats,” stated Hugh Rogers, CEO of XPhyto. “We see an enormous global market opportunity that includes individual households, schools, hospitals, public transportation, airports and border services as well as many private employers.”
Patients infected with an alternate coronavirus strain or a highly mutated form of COVID-19 are expected to activate only the universal coronavirus probes. These patients could be selected for further investigation.
XPhyto’s next developmental milestones:
- Design and manufacture of screening tests
- Initiation of clinical evaluation protocols
- A pilot study using human saliva from healthy and COVID-19 infected patients.

Some investors expressed skepticism about efficacy of saliva-based swabbing.
The jury is still out, although recent medical studies back up XPhyto’s theory.
“Researchers from Rutgers University evaluated the use of salivary specimens for SARS-CoV-2 detection in symptomatic patients from three ambulatory care centers.
reported The Journal of Sciences and Specialties of The Head and Neck, “The data, although limited, suggest that saliva may be more sensitive in detection of asymptomatic or pre-symptomatic infections”.
In other words, XPhyto’s test may be ideal to catch the super-spreaders – the infectious carriers blithely attending drumming circles because they feel healthy.
“A salivary diagnostic test would allow for a non-invasive self-https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered sample collection under health-care providers’ directive for individuals in quarantine,” confirmed the report, “and would circumvent the issues regarding global shortage of swabs.”
Conclusion: Saliva may be a viable alternative to nasopharyngeal specimen for COVID‐19 testing.

Sona Nanotech (SONA.C) has also been doing good work in this arena – announcing that its “rapid detection, COVID-19 antigen technology resulted in a test sensitivity of 96%, test specificity of 96%” in a lab with cultured virus.
Sona stated that “sales of the tests will now be permitted under a ‘research use only’ label until full regulatory authority is granted.”
Both Sona and XPhyto have good-looking charts, but it’s fair to say that Sona’s stock price has received more love than XPhyto’s.
In a July 24, 2020 article on Stockhouse, veteran financial journalist Marc Davis [USA Today, CBS Money Watch, National Post, Barron’s, China Daily etc.] did an excellent job of comparing-and-contrasting Sona and XPhyto.
“What both of these very different companies seem to have in common is the technological knowhow to develop rapid, disposable diagnostic tools that are well-suited to tackling the global pandemic,” stated Davis.
“Sona saw its share price catapult as much as 175% from CDN $3.13 a share to $8.65 after announcing validation results for its Covid-19 antigen test earlier this month. This was a hotly-anticipated news release and its findings prompted a buying frenzy among investors, triggering heavy volume. The stock is now trading well above $10.00 a share,” continued Davis
“XPhyto is a little further behind the developmental curve than its competitor,” stated Davis, “Ironically, its buoyant share seems to have little to do with its R&D work in infectious diseases. Instead, the stock has run from an IPO last year at CDN $0.50 to around $3 on the back of news developments almost entirely related to XPhyto’s other R&D projects”.
Davis makes an excellent point here.
XPhyto is a fire-breathing multi-headed dragon, with a pipe-line of projects which includes a focus on Parkinson’s Disease and CBD delivery systems.
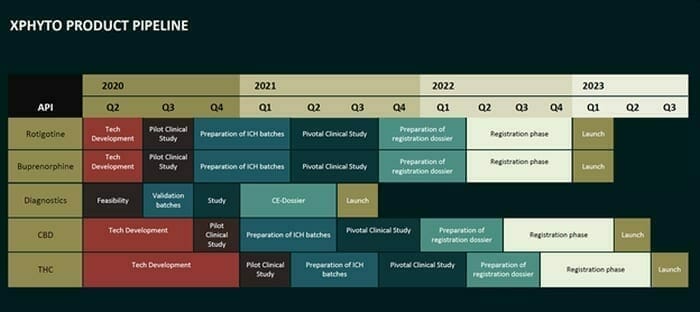
This is a common curse of ambitious small-cap stocks with a lot of balls in the air.
In the cluttered minds of harried retail investors, the company becomes a blurred levitating shape.
With XPhyto, you also have the issue of the CEO who may be too modest for his own good.
“We have been intentionally low-key about what we are doing, instead choosing to focus on communicating our other business verticals in Germany,” stated XPhyto CEO Hugh Rogers.
“This leaves plenty of upside potential for XPhyto if its share price is to catch up with Sona in the coming months,” observed Davis.

“Further to its successful prototype testing, XPhyto is now gearing up for a short pilot study lasting less than 30 days, and involving testing human saliva from healthy patients and saliva spiked with COVID-19 RNA in its lateral flow assay devices” stated Davis.
“The fast results from an antigen test mean that people who test positive can be isolated quickly, before they risk infecting others,” states Science Mag, “Even if the tests have a 10% false negative rate, “people could easily be tested repeatedly, making it likely that anyone missed on the first round would be flagged on the second.”
“The global economy could take a hit of some $82 trillion in a worst case scenario from the coronavirus,” according to the University of Cambridge, “In case of speedy recovery, an “optimistic loss” of $3.3 trillion is likely.”
“Testing is the biggest problem that we’re facing,” confirmed Peter Slavin, president of Massachusetts General Hospital in a roundtable on Covid-19 at Harvard Medical School.
XPhyto is making significant progress solving this problem.
XPHY’s market cap is currently $165 million.
If that is too low, it’s likely because the market is distracted by the ambient noise of its auxiliary businesses.
Full Disclosure: XPhyto is an Equity Guru marketing client.


Leave a Reply