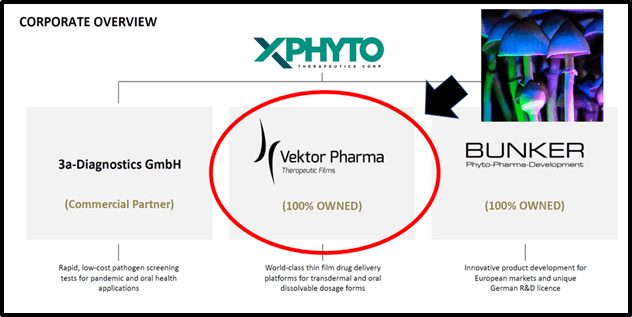
On November 3, 2020 Xphyto Therapeutics (XPHY.C) announced that its 100% owned German subsidiary, Vektor Pharma, has signed a research agreement with a leading German university for “the exclusive development of a proprietary biotechnology process for the industrial manufacture of psilocybin as a certified active pharmaceutical ingredient (API)”.
Psilocybin is a natural psychedelic compound produced by 200 species of fungus. The fungal mushrooms have hallucinogenic effects when eaten.
“Psychedelic agents are a promising new class of therapeutic drug,” stated Hugh Rogers, CEO and Director of Xphyto, “We see a natural opportunity to apply XPhyto’s drug formulation expertise and Vektor’s proven drug delivery platforms to these emerging APIs.”
The timing of this announcement is prescient.
There’s a federal election today south of the border, to decide the 46th President of the U.S.
Only 16% of Canadians approve of Donald Trump.
The other 84% of Canucks practice political activism by posting “tiny-hand-Trump-memes” on Facebook – receiving many “likes” and “hearts” from their peer group.
“Left-leaning Canadians on FB inspired me to become a democrat” – said No Trump Supporter Ever.

Mushrooms are decriminalized in four U.S. cities, but Psilocybin is listed in Schedule I of the Controlled Substances Act, “making it illegal to cultivate or possess psilocybin producing mushrooms for either personal consumption or distribution.”
Today’s federal election results could change the regulatory landscape in Oregon and Washington DC, as the residents of those states will vote to decide if psilocybin should be decriminalised.
Initiative 81 would make arrests for cultivation and use of psychedelic plants “a low law enforcement priority” for D.C. police.
Oregon Measure 109, the Psilocybin Mushroom Services Program Initiative, proposes legalizing it for medical purposes and establishing a plan for https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistering the drug in clinical settings.
Xphyto has identified a number of psychedelic compounds that are emerging as strong potential candidates for the treatment of mental health related medical indications such as depression, anxiety, addiction, anorexia and post-traumatic stress disorder.
The US Food and Drug Administration (FDA) has twice designated psilocybin as a “breakthrough therapy” for the treatment of severe and treatment-resistant depression.
It’s not difficult to see how Xphyto’s existing technology could be deployed in an arena where bio-availability and dosage-control will be paramount.
Six weeks ago, Xphyto announced that Vektor Pharma is advancing two sublingual Oral Dissolvable Film (ODF) development programs to deliver cannabidiol (CBD) and tetrahydrocannabinol (THC).
Oral Dissolvable Film (ODF) sub-lingual (oral) strips are known to increase bioavailability. It’s an upstream medical technology that can be applied to a wide array of different drugs.
XPhyto intends to secure industrial scale production of psychedelic APIs and the standardization of drug formulations for the delivery of such APIs.
The biotechnology production development program began in October 2020 with completion expected in September 2021.
In the video below, Hugh Rogers, CEO of Xphyto explains the rationale for the Vektor Pharma acquisition and his business objectives with this acquisition.
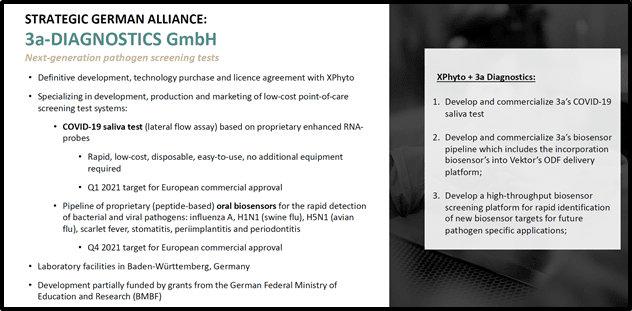
Meanwhile this summer, Xphyto announced that its exclusive diagnostic partner, 3a-Diagnostics took a major step towards the development of an affordable, portable screening tool that will disrupt the fight against pandemic threats.
3a is a German research-based biotech that specializes in point-of-care test systems.
The July 6, 2020 news confirmed successful function of 3a’s proprietary coronavirus RNA probes, though prototype Lateral Flow Assay (LFA) testing.
“LFAs are designed for use at point of care/need: the physician’s examination room, emergency ward in general hospitals,” explains the Analytical and Bioanalytical Chemistry Journal.
“Especially in third-world countries these tests are used for biomedical purposes, since there is no need to refrigerate the strips,” continues the Journal, “Owing to their extended shelf life, large batches can be prepared, diminishing the variation between batches”.
LFAs are designed for disposable single use so that no contamination with a previously tested sample will occur.
The most famous application is the home pregnancy test.
“We believe that a low-cost, portable and easy to use screening tool that provides rapid on-the-spot results would be a disruptive tool in the fight against pandemic threats,” stated Rogers. “We see an enormous global market opportunity that includes individual households, schools, hospitals, public transportation, airports and border services as well as many private employers.”
Patients infected with an alternate coronavirus strain or a highly mutated form of COVID-19 are expected to activate only the universal coronavirus probes. These patients could be selected for further investigation.
XPhyto’s current developmental milestones:
- Design and manufacture of LFA screening tests
- Initiation of clinical evaluation protocols
- A pilot study using human saliva from healthy and COVID-19 infected patients.
“Our goal is the development of precise and predictable psychedelic drug formulations for clinical study and therapeutic use,” stated Rogers about the November 3, 2020 announcement.
Xphyto will provide an update on the psilocybin production program with milestones and timelines in the coming weeks.
“50 years I’ve been bumbling around this planet, and the best damn weekend I’ve had on it to date occurred recently, under the influence of mushrooms,” wrote Equity Guru’s Chris Parry on December 9, 2020.
“The carpet didn’t talk to me and the walls weren’t bleeding, and the sunrise wasn’t the incarnation of Shiva. That ain’t it, kids,” added Parry, “No, what happened is my head just opened right up and someone I cared about did likewise and a relationship went to a place it hadn’t before and everything changed going forward.”
This morning, President Trump admitted that he is “not thinking about concession speech or acceptance speech yet.”
“Winning is easy,” added Trump, “Losing is never easy.”
For the tortured, toxic, Trump, a dose of psilocybin might “open his head up” so that he can accept losing easier.
The global antidepressant drugs market is predicted to reach approximately USD $16 billion by 2023, with an estimated CAGR of 2.1% from 2017 to 2023 according to Allied Market Research.
- Lukas Kane
Full Disclosure: Xphyto is an Equity Guru marketing client


Leave a Reply