On November 11, 2020 Revive Therapeutics (RVV.C) Revive Therapeutics updated shareholders on its oral thin-film delivery system with psilocybin being developed “under a research partnership agreement with Reed Research Group out of the University of Wisconsin-Madison”.
RVV is a specialty life sciences company focused on the R&D of therapeutics for medical needs and rare disorders.
“Psilocybin has been shown by Functional Magnetic Resonance Imaging (fMRI) to create a state of hyperconnectivity between brain networks,” states Double Blind Magazine.
Political winds are shifting.
Equity Guru readers who invested in cannabis in 2017 know how profitable it can be to ride a macro-investment wave driven by a changing regulatory environment.
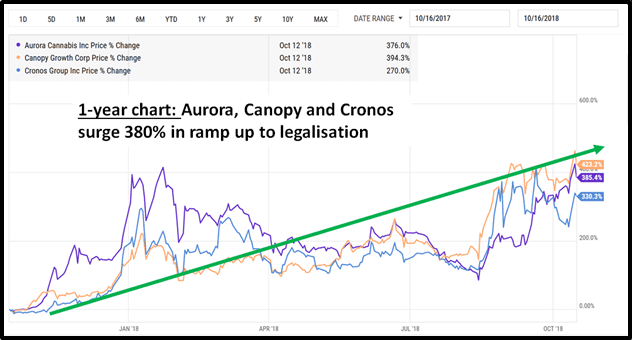
“On Nov. 3, 2020 the state of Oregon voted to legalize psychedelic mushrooms for therapeutic use, a first for the nation,” writes a Quartz Magazine Health and Science Reporter, “And residents of Washington DC passed a ballot initiative that would decriminalize magic mushrooms and other psychedelics”.
“The initiatives show support for loosening drug laws on psychedelics,” continues Quartz, “and sow the seeds for two potentially lucrative psychedelic markets: a recreational one and a medical one”.
Key Features of current RVV prototyping and dosage research:
- Dosage forms ranging between 1 mg and 20 mg
- Diversity through physio-chemical characterization
- Tensile strength of films is being tested
- Bio comparable tannin-chitosan composite materials
- Dissolution and disintegration testing
- Rate of psilocybin release from composites.
- Technical and scientific data is being processed and finalized.
“Our orally dissolvable thin film strip for psilocybin, can be used in FDA human clinical studies and as a unique product for medical use in states where psilocybin therapy use is permitted, such as Oregon following the passage of Measure 109,” stated Michael Frank, CEO of Revive.
“There is a significant market opportunity for our unique oral thin film strip technology for not only delivering psilocybin but also delivering numerous psychedelic-based medicines to treat various diseases and disorders that would benefit from such a delivery method,” continued Frank.
RVV plans to partner with life sciences companies seeking to add unique offerings in their psychedelic-based product pipeline and with companies operating in the U.S. where psilocybin therapy use is legal.
Advantages of the orally dissolvable psilocybin thin film technology:
- Rapid dissolving and onset of action to the bloodstream
- Ease and convenience for patients to https://equity.guru/wp-content/uploads/2021/10/tnw8sVO3j-2.pngister
- No need for water, chewing or swallowing
- Potential for improved therapeutic outcomes
- Efficacy for underserved diseases
- Flexibility to create accurate dosing
RVV is also exploring the use of Bucillamine for the potential treatment of infectious diseases, with an initial focus on severe influenza and COVID-19.
Bucillamine is a versatile organic molecule with an array of utilities including “regulating B-cell function” “thiol antioxidant” and “cisplatin-induced otoxicity”.
“Bucillamine has potential to attenuate or prevent damage during myocardial infarction, cardiac surgery and organ transplantation,” states the University of Colorado Health Sciences Center, it “enters the cells by the same mechanism that normally transports the amino acid cysteine”.
Preclinical and clinical studies have demonstrated that bucillamine can significantly reduce the negative symptoms of respiratory viral infections in animals and humans.
Revive has identified bucillamine as potentially useful in the treatment of COVID-19.
With the FDA’s approval, Revive has set-up five clinical sites in Florida, Texas and California for enrolment of patients in the phase 3 clinical study.
It is also finalizing agreements with an additional 10 clinical sites in these states, including Arizona and Ohio.
Revive’s cannabinoid pharmaceutical portfolio focuses on rare inflammatory diseases and the company was granted FDA orphan drug status designation for the use of Cannabidiol (CBD) to treat autoimmune hepatitis (liver disease) and to treat ischemia and reperfusion injury from organ transplantation.
Three weeks ago, RVV signed a supply agreement with Havn Life Sciences (HAVN.C) to source naturally-derived psychedelic compounds, such as psilocybin, for use in future investigational new drug enabling studies and clinical trials under FDA guidelines.
“We are excited about our strategic partnership with Havn Life as one of our suppliers of psychoactive compounds that we intend to develop and commercialize using our established tannin-chitosan based proprietary oral-thin film delivery system, for the pharmaceutical and wellness markets,” stated Frank.
We are thrilled to have signed this supply agreement with Revive Therapeutics to help further their work in this field,” added Susan Chapelle, Co-CEO, Havn Life, “Both of our companies are leading innovators in the space, and we look forward to helping Revive achieve their goals with our compound supply.”
“Right now, Revive is one of the early entrants to the burgeoning shrooms/psychedelics/psilocybin industry, a business that is so early it’s not yet legal,” wrote Chris Parry on March 20, 2020, “You could roll your eyes at that, but there are plenty of indicators to suggest the shroom movement will land quicker than medical marijuana did”.
Full Disclosure: RVV is an Equity Guru marketing client.


Leave a Reply