+135k shares in a downturn, my man
The psychedelics sector is down bad this week.
Just look at the psychedelics ETF PSYK.NE.
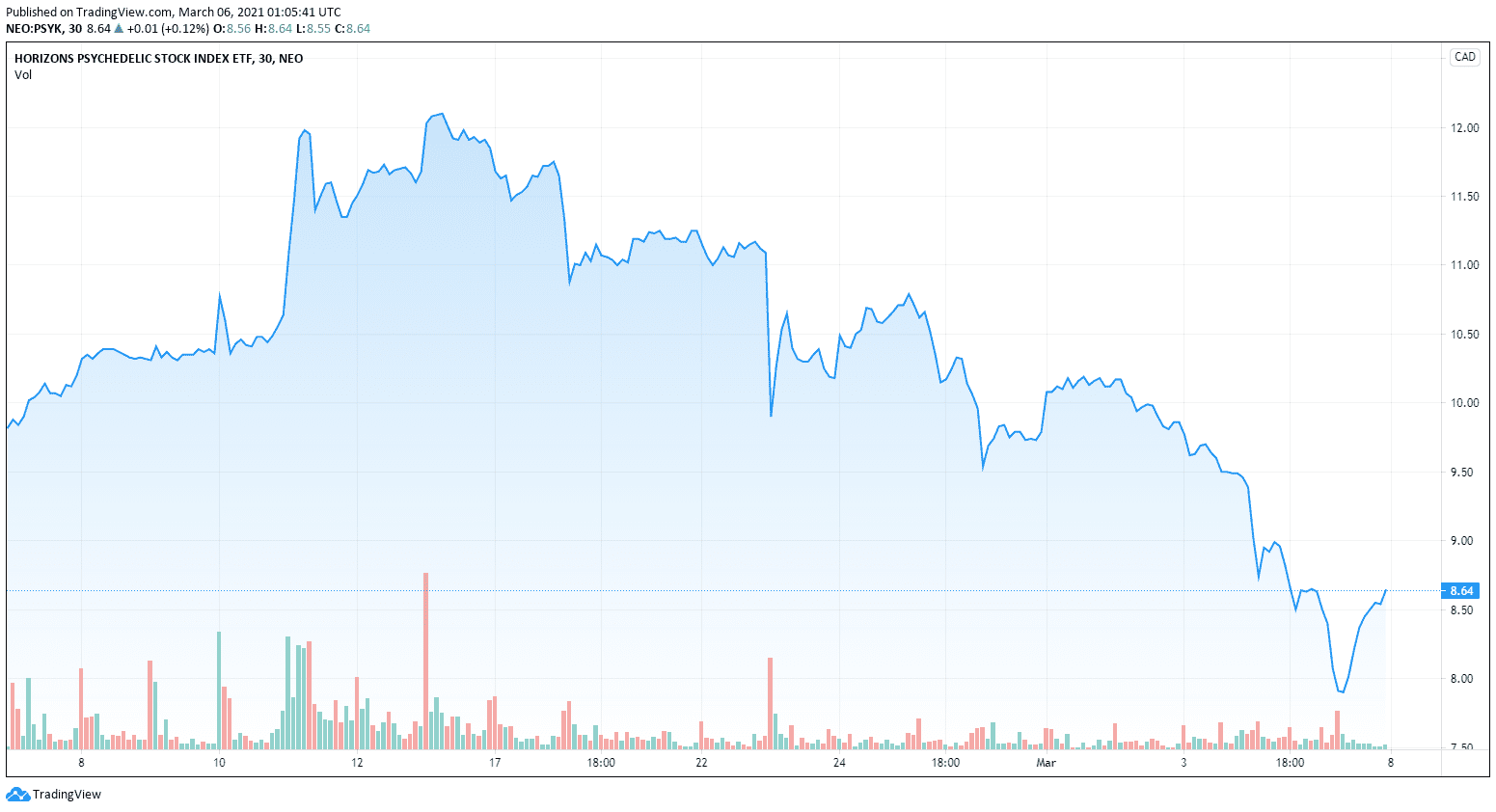
It’s not an alarm or anything, things have been running pretty nicely the past couple of quarters and it was probably time for a correction.
Any time a sector emerges this quickly there is going to be a lot of volatility, speculative investors need to have strong stomachs and not panic at unpredictable price swings.
Most investors long on psychedelics are waiting for significant policy changes with regards to psychedelic compounds, selling before that happens wouldn’t make much sense. My read is that 95% of investors in the sector are still confident.
One thing that does instill investor confidence is insider buying, especially during a downturn – and that’s what we are seeing this week with Revive Therapeutics CEO Michael Frank picking up 135k shares in the open market on a down week, increasing his position from 1,811,500 shares to 1,946,500 shares.
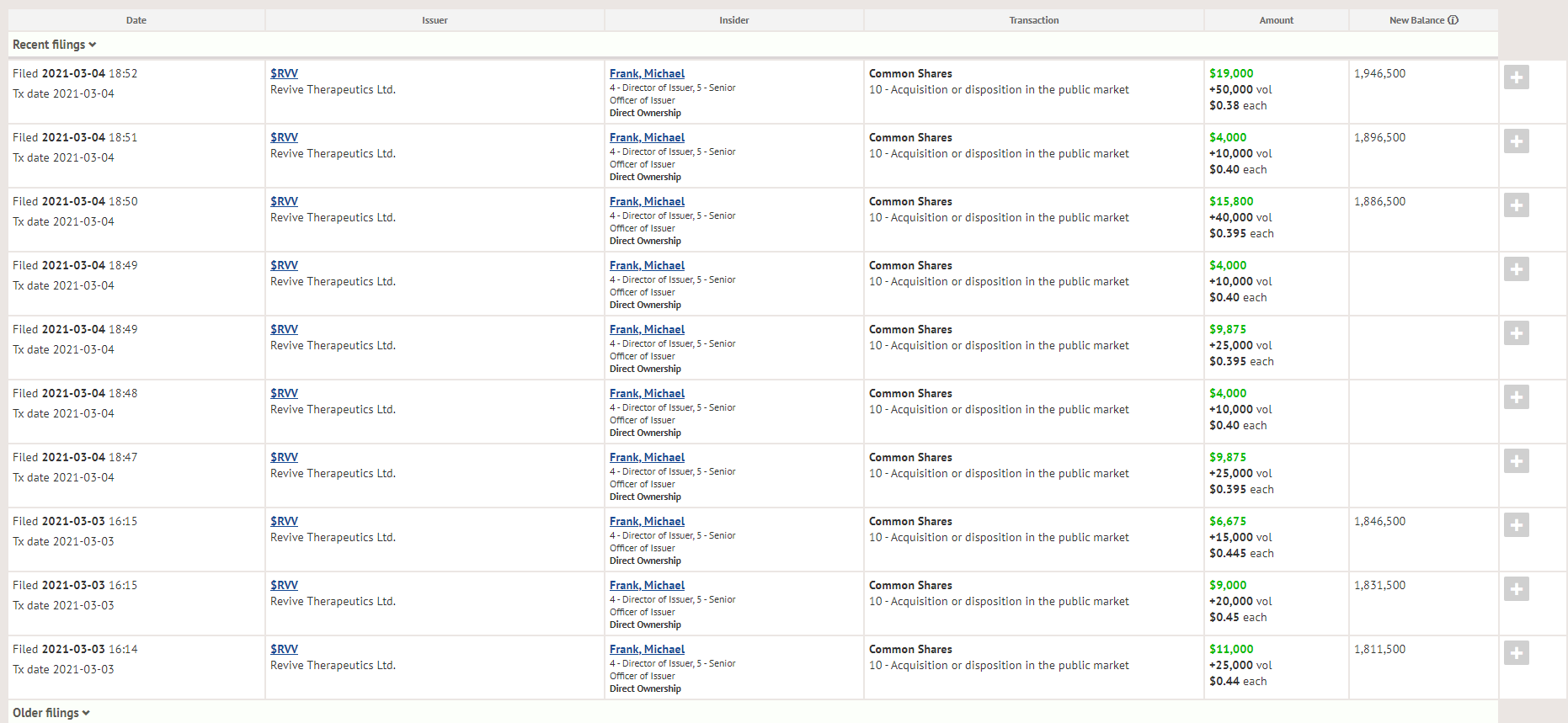
Insiders sell the stock for a number of reasons, usually to either scale the company – or line their own pockets, but they only buy stock for reason – they think it’s going to go up.
Last week I wrote about the importance of being on the same page as management and investing in companies where management is significantly motivated to bring the share price up.
In doing some research it’s easy to find management who are holding roles at multiple companies, this is especially common in cannabis and mining. It’s important to buy into a company that is actively marketing itself and not just a shell floating around.
This is a common mistake newer investors make, hell, you might even be more invested than management if we are talking about some of the weed penny stocks on the CSE.
Revive, on the other hand, looks to be bought in.
Investors were happy about the buying.
Phase III expansion
Revive has a lot to look forward to.
After a recent upsized 23M raise the company in a position to make moves. With a thick pile of patents, active clinical trials, and a pipeline of IP with exposure to psychedelics, cannabis and COVID treatments Revive is in all the right places for what’s going on in the world right now.
Revive is in phase 3 of its FDA clinical trials of Bucillamine for COVID symptoms. Known for its powerful anti-inflammatory and anti-oxidant benefits, Bucillamine has been prescribed for decades in countries like Japan and South Korea.
Revive didn’t just jump on Bucillamine when COVID hit, the company tested the impact of Bucillamine on gout in 2015 in a Phase 2 FDA study. The results met primary efficacy and safety endpoints for the treatment of gout, however, Revive put the project on hold to focus on its cannabidiol trials.
To date, Revive has completed treatment on 210 of the 1000 total patients for their phase 3 study on Bucillamine. The Data Safety Monitoring Board gave Revive the go-ahead to expand the number of trial sites from 14 to 50, so there could be something promising coming down the line as that is a significant expansion.
The development of a useful therapeutic treatment for COVID-19 would be substantial, especially as vaccine rollouts in richer countries have been extremely delayed in many instances, and several lower-income countries are struggling to find a solution.
The Phase 3 confirmatory clinical trial titled, “A Multi-Center, Randomized, Double-Blind, Placebo-Controlled Study of Bucillamine in Patients with Mild-Moderate COVID-19”, will enroll up to 1,000 patients that will be randomized to either Bucillamine or placebo for up to 14 days. The primary objective is to compare the frequency of hospitalization or death in patients with mild-moderate COVID-19 receiving Bucillamine with those receiving placebo.
Overall 2021 outlook is kinda…bleek, but Revive looks good!
The company has several milestones in psychedelics, COVID treatments, and cannabis coming up this year.
For psychedelics, they are scaling up their psilocybin oral film strips and posting their research results of psilocybin for concussions, while also beginning phase I of their psilocybin for meth addiction study with the University of Wisconsin-Madison.
They plan to complete enrollment of their phase III COVID study by Q2 of this year, and will also be initiating phase II of their CBD study for autoimmune hepatitis. 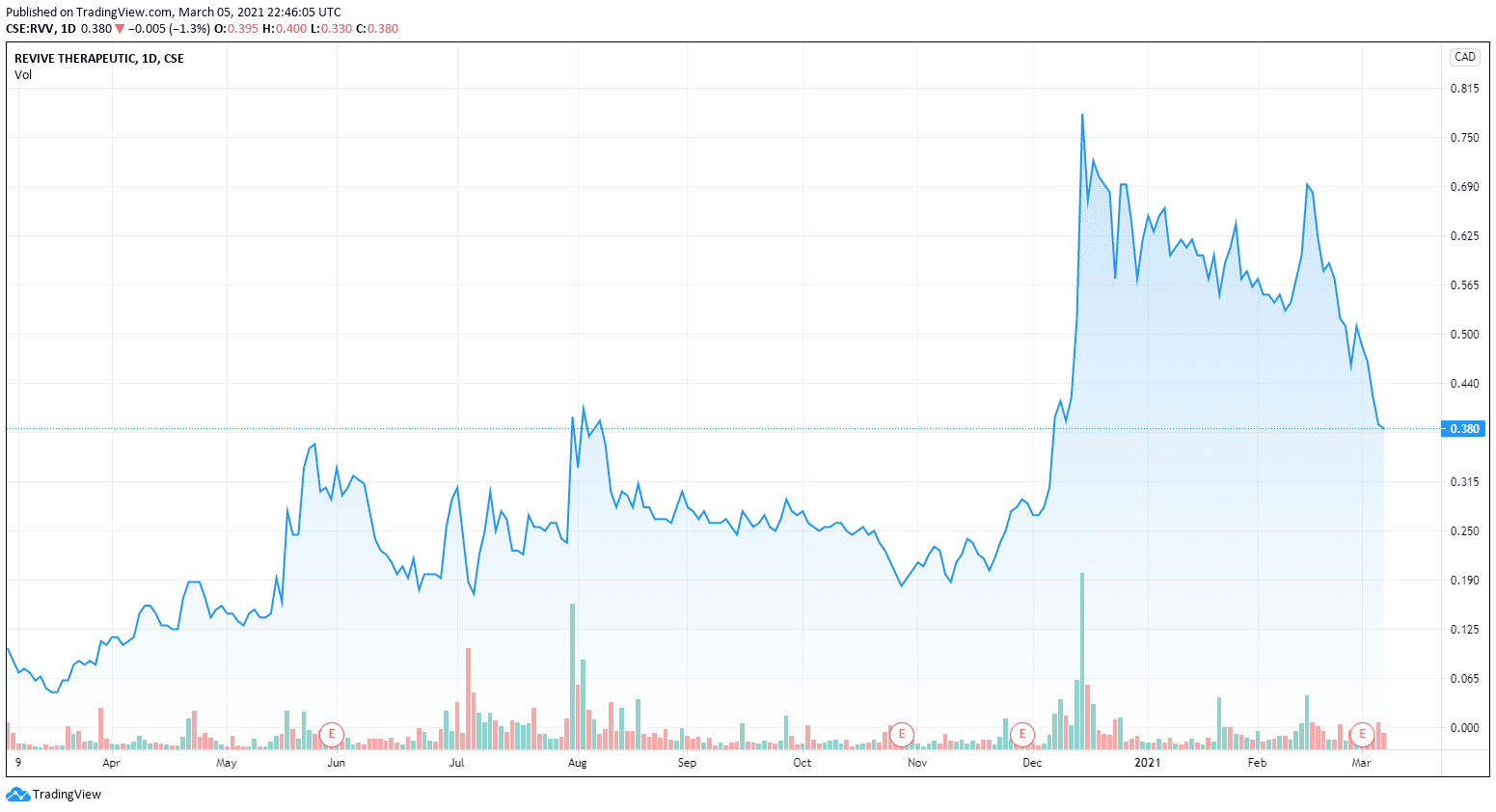
Investors continue to be bullish on Revive as an undervalued sleeper company at only a $71M CAD market cap. Their diverse portfolio has a lot for investors to get excited about. Last month I wrote about how the company has built itself to have several kicks at the can, several kicks at several cans actually.
Stocks around the psychedelics sector have all taken a slide over the past month or so. If investors are bullish on the sector this could be a great time to pick up shares as no real news or market change has affected this. It looks like a simple correction that happens with all highly speculative sectors.
Getting into a sector this early usually means the dips in the overall market are less relevant to the actual companies and what they are producing, and more about market speculation. It’s kind of a weird time because so much of the valuation is based on future projections, we saw this in cannabis as well, but it’s where the most money is made.
Full disclosure: Revive Therapeutics is an equity.guru marketing client.


Leave a Reply