Ortho Regenerative Technologies (ORTH.C) is focused on regenerative technologies, which will dramatically improve the success rate of orthopedic and sports medicine surgeries.
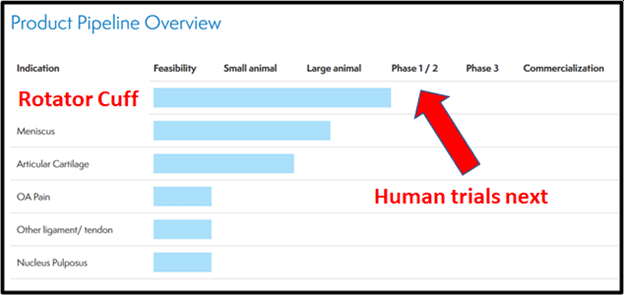
On April 6, 2021 ORTH announced that it has submitted an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) for the initiation of a Phase I/II clinical trial of ORTHO-R in rotator cuff tear repair.
“The IND submission brings us one step closer to the enrollment of our first patient, a recognized value creation event in our industry”, stated Claude LeDuc, President and CEO of Ortho RTI. “There is a clear need for improved rotator cuff tear repair treatments, as estimates have put the re-tear (or non healing) rate at an average of 50%.
“With more than 600,000 patients undergoing rotator cuff surgery every year in the U.S., this represents an enormous commercial opportunity, and as demonstrated in 2020 by our GLP preclinical program results,” added LeDuc, “We strongly believe that ORTHO-R can help address these significant unmet needs and meaningfully improve the success rate of these surgeries.”
ORTH’s growth story has two underpinnings: the first is surging healthcare spending.
According to IHS Market, Global healthcare spending was an astonishing USD $8.3 trillion in 2020 and will grow 5.8% to USD $8.8 trillion in 2021.
The second part of the growth story is the expanding ranks of physically active seniors.
Between 2016 and 2021, worldwide life expectancy rose from 73 years to 74.1 years. The number of people aged 65 or older is projected to grow from an estimated 524 million in 2010 to nearly 1.5 billion in 2050.
These feisty old bastards refuse to act their age.
Here in Vancouver, BC, the BC Older Timers Soccer League has 30 teams registered in Over-55, and Over-60 category.
These dudes play hard, and their bodies are prone to injury.
“A unique quality of the newly aging group of people referred to as baby boomers is their expectation to continue exercising as they grow older,” states the American Journal of Roentgenology, “thus the incidence of exercise-induced injuries among older people is increasing”.

Ortho RTI is dedicated to the development of novel therapeutic tissue repair technologies.
The proprietary technology platform, is a muco-adhesive CHITOSAN based biopolymer matrix, specifically designed to deliver biologics such as Platelet-Rich Plasma (PRP) or Bone Marrow Aspirate Concentrate (BMAC), to augment and guide the regeneration of new tissue in various musculoskeletal conditions.
In ORTH’s product pipe-line, Ortho-R – a Chitosan-PRP hybrid biologic implant combination product, is designed to increase the healing rates of occupational and sports related injuries to tendons, meniscus and ligaments.
Other formulations are being developed for cartilage repair, bone void filling and osteoarthritis treatment.
Value proposition
- Addressing significant unmet market and medical needs
- Need for improving standard of care surgery outcomes
- First solution to address the lack of residence time of Orthobiologics use in musculoskeletal conditions
- USD $5 billion soft tissue repair global market
Novel Technology
- Increases very significantly the residence time and impedes blood clot retraction of Orthobiologics use in the Treatment of Soft Tissue Injuries in Various Musculoskeletal Conditions
- Validated mode of action, safe and easy to use solution, adjunct to standard of care surgery
- New therapeutic areas in development
- Smart Business Model
A Clinical Stage Company focusing on:
- Developing Orthobiologics applications in US Market first
- High volume, low-cost products
- Reducing healthcare stakeholders’ costs
- Improving standard of care surgery outcomes
ORTH’s tech is designed to improve surgical outcomes for the kinds of injuries that are increasing in an aging but still active population.
The joints with the most 3D-rotation often require surgery (backs, knees, shoulders).
ORTH has three patent families:
‘782 Family#1 (composition of matter):
- polymer composition and PRP
- Patent granted under the law of United States on August 30, 2016.
- Patent granted under the law of European Union
PCT ‘129 Family#2 (composition of matter):
- freeze dried chitosan and lyoprotectant for use with PRP, blood and
- combinations to form an injectable solution
- currently being prosecuted
- Australia granted
PCT ’13 Family #3 (composition and use)
- Lyophilized scaffold comprising at least one polysaccharide, to have a variety of beneficial effects for tissue repair.
- currently being prosecuted
This Equity Guru video gives an overview of ORTH’s technology, and its commercial applications.
“Soft tissue injuries such as sprains, strains and tendinitis result in nearly 70 million physician office visits a year in the US alone, creating an annual USD $54 billion economic burden.”
“With an aging population expected to increase 50% by 2030, and an average 50% failure rate on such interventions as rotator cuff surgeries, both patients and practitioners are desperate for a better way to ensure a successful recovery from serious soft tissue damage.”
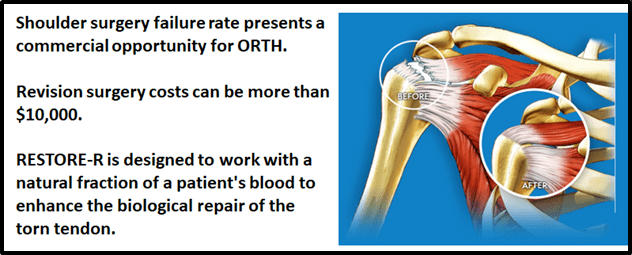
“ORTH’s proprietary restore technology platform has hit clinical stage testing for use in the over 600,000 rotator cuff surgeries carried out each year in the U.S.”
“The global soft tissue repair market is expected to reach $10.7 billion by 2025,” states Grandview Research.
“There are over 600,000 rotator cuff surgeries being performed in US each year, and the number is rising at approximately 5%,” states ORTH, “Not all of these surgeries are successful: the success rate for surgery is dependent on many factors including the size of the original tear and the age of the patient. Estimates have put the re-tear (or non healing) rate at an average of 50%.”
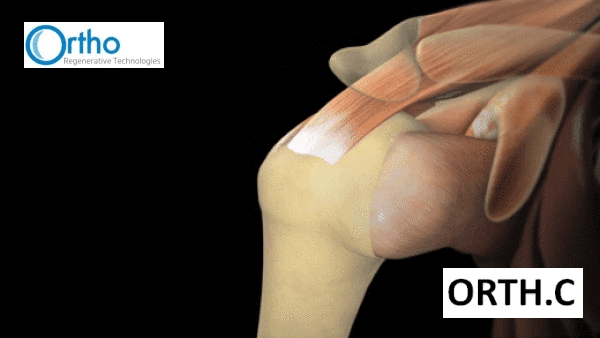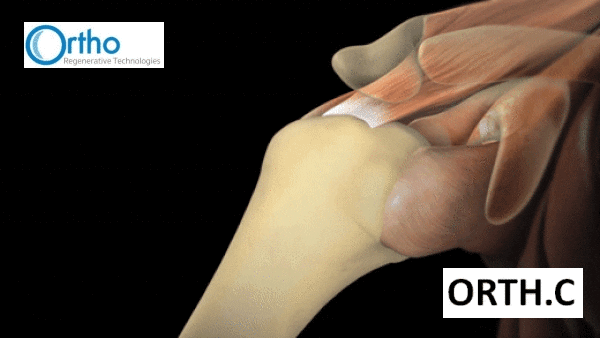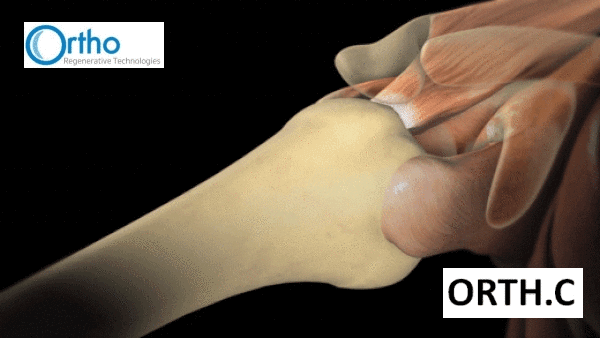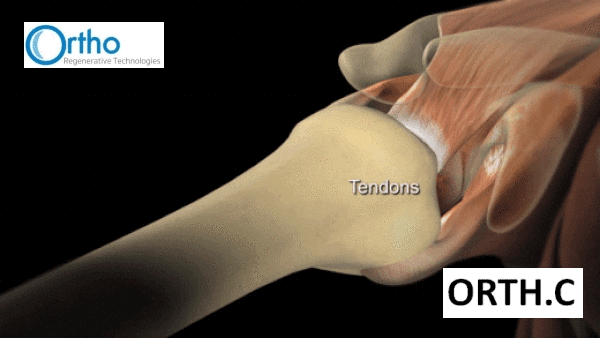
“The rotator cuff is made up of four muscles and tendons that anchor the head of the upper arm bone securely into the socket of the shoulder joint. The muscles of the rotator cuff pull on bands of tough in elastic tissue called tendons to raise and rotate your arm while keeping the shoulder joint stable.
“Your rotator cuff can be injured by falling, lifting, pulling or doing repetitive overhead arm motions.”
On March 2, 2021 Ortho RTI provided a review of is 2020 achievements and future milestones as ORTH transitions from preclinical to clinical stage.
2020 Achievements
- Completed four non-brokered private placements that raised an aggregate C$6.7 million. The company now has more than $2 million cash on hand to fund its regulatory and clinical stage projects through 2022.
- In March and July, Ortho RTI announced positive preclinical results that confirmed the safety profile and demonstrated statistically significant improvement over standard of care for rotator cuff repair.
- Following a request for designation to the FDA, ORTHO-R was classified in August as a drug/biologic, which management believes will enhance its long-term market potential.
- In October, the Company appointed Mukesh Ahuja, MBBS, MSc as Vice-President Clinical and Medical Affairs, who brings extensive expertise in orthobiologics clinical development.
- In October, Ortho RTI shares began trading on the OTCQB market, facilitating better access for US investors and improved trading liquidity.
- In November, Ortho RTI initiated the scale-up and manufacturing of cGMP clinical trial material to be used in the upcoming ORTHO-R Phase I/II rotator cuff tear repair clinical trial in the US.
Achieved and Planned Milestones 2021-2022
- In January 2021 Ortho-RTI entered into a global licensing with Hanuman Pelican, Inc. for the rights to commercialize the Buoy Suspension Fractional System, one of the best PRP systems available, in combination with ORTHO-R.
- The Company made changes to its Board of Directors in February designed to better position the Company for its next phase of growth.
- Completion of ORTHO-R cGMP clinical trial material manufacturing is expected in March.
- The Company plans to submit an Investigational New Drug (IND) Application for ORTHO-R in March of 2021, and to begin enrolling our multi-center Phase I/II trials in the US in Q2 2021
- Select US investment bankers in the second half of the year.
- Planning of ORTHO-R meniscus indication in the second quarter of 2021, with the start of a pivotal preclinical trial for ORTHO-R second soft tissue repair indication
- Begin preparations in 2022 for Phase III trials in the US, Canada, and EU planned for 2023.
- The Company plans to opportunistically evaluate additional potential partnerships throughout 2021 and 2022.
“We now have adequate financing for the next 12-15 months – which includes the start of our upcoming multi-center Phase I/II trials in the United States,” states Claude LeDuc, President and CEO of Ortho RTI, “We are focused on the clinical development of ORTHO-R.”
“2021 will be transformational for the company as we advance the clinical development of our lead product,” added LeDuc.
The Phase I/II clinical trial announced April 6, 2021 is a prospective, randomized, controlled and blinded study to evaluate the safety and efficacy of ORTHO-R + standard of care surgery vs standard of care surgery alone in rotator cuff tear repair.
The clinical trial will enroll a total of 78 patients at 6 to 10 clinical sites throughout the U.S. Enrollment is expected to begin in Q2 of 2021.
Lukas Kane
Full Disclosure: Ortho Regenerative Technologies is an Equity Guru marketing client.

Leave a Reply