On April 20, 2021 XPhyto Therapeutics (XPHY.C) announced two significant appointments to its operations team, as Europe passed “the grim milestone of one million coronavirus deaths, with infections rising exponentially.”
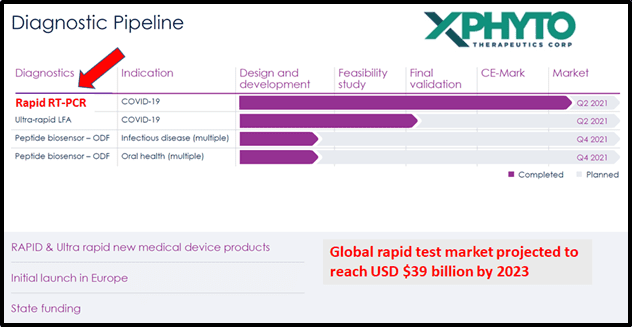
Xphyto – a bioscience accelerator – has completed validation of the Covid-19 PCR Diagnostic Test Kit.
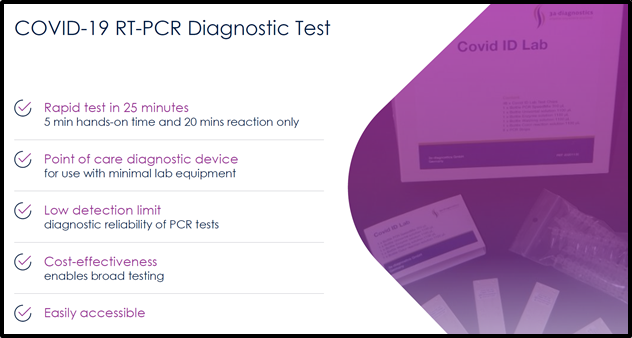
The PCR test system demonstrated clinical accuracy in detecting SARS-CoV-2 RNA within 25 minutes, making it an ideal screening tool for international airports.
Xphyto’s Q2, 2021 commercial roll-out is timely.
Mr. Wolfgang Probst is now the Chief Operations Officer (COO) of XPhyto.
Mr. Manfred Buchberger is now the Head of Corporate Development at XP Diagnostics GmbH (XP Diagnostics), a 100% owned German subsidiary of XPhyto.
These new-talent announcements are often ignored by retail investors, but they can be important drivers of shareholder value.
“The late Steve Jobs of Apple summed up talent’s importance with this advice: “Go after the cream of the cream. A small team of A+ players can run circles around a giant team of B and C players.” reported Mckinsey & Co.
Management guru Jim Collins concurred: “the single biggest constraint on the success of my organization is the ability to get and to hang on to enough of the right people”.
XPhyto has a track record of attracting top talent.
Two months ago, XPhyto announced that it has fast-tracked the assembly of an elite commercial team to launch its point-of-care COVID-19 RT-PCR test system.
“PCR tests are used to directly detect the presence of an antigen, rather than the presence of the body’s immune response, or antibodies,” states Medical Device Network, “By detecting viral RNA, which will be present in the body before antibodies form or symptoms of the disease are present, the tests can tell whether or not someone has the virus very early on.”
As a 2nd wave of Covid-19 sweeps across the globe, commercial product launch in Europe is planned for Q2, 2021.
Xphyto is a diversified bioscience company.
Mr. Probst is an experienced management and finance consultant with expertise in mergers and acquisitions, corporate re-organizations, and divestitures.
He has a proven track record of leadership, strategic planning, and organizational restructuring having guided multiple startups from inception to financial success.
Mr. Probst was responsible for successfully structuring XPhyto’s European operations and establishing associated partnerships and collaborations, both commercial and academic.
As the newly appointed COO of XPhyto, Mr. Probst will be responsible for managing the Company’s global operations. Mr. Probst will remain a director of XPhyto and the managing director of XP Diagnostics.
Mr. Buchberger is a global leader in the medical diagnostics industry. For almost a decade, he was the CEO and a member of the Global Management Board of a major European medical diagnostics and analytics company with annual revenues of over 600 million Euro.
His addition to the Company brings decades of diagnostics industry experience focused on international business development, sales, marketing, and product management.
Mr. Buchberger has successfully created, implemented, and managed business growth strategies across Europe, North and South America, Middle East and Asia, including the successful launch of new medical diagnostic products.
On March 18, 2021 Xphyto’s exclusive German diagnostics development partner, 3a-diagnostics GmbH (3a), announced they have received approval for their point-of-care Covid-19 rapid RT-PCR test system (Covid-ID Lab) for use in Europe.

After receiving their ISO certification, XPhyto and 3a’s Covid-ID Lab is now registered within the European Union as a commercial in vitro diagnostic (CE-IVD) test.
Xphyto sent out thousands of tests to potential distributors.
The Covid-ID Lab will allow people to receive rapid test results, getting their results back after as quick as 25 minutes, and has a 95% confidence interval.
“Our test is one of the fastest PCR-based COVID-19 tests currently approved. With a sample collection to result time of 25 minutes, Covid-ID Lab combines the speed of a rapid screening test with the accuracy of a PCR diagnostic,” stated Hugh Rogers, CEO and Director of XPhyto. “Covid-ID Lab is designed for point-of-care testing, particularly in satellite and small-scale labs, such as transportation hubs, borders, care facilities, schools, pharmacies, and hospitality settings.”
“The death toll across Europe’s 52 countries, compiled by AFP from official sources, totalled at least 1,000,288,” reported France 24.
“We are in a critical point of the pandemic right now,” confirmed Maria Van Kerkhove, the WHO’s technical lead on Covid-19.
“The trajectory of this pandemic is growing… exponentially”.
The market for Xphyto’s technology is global.
“India is struggling to cope with the soaring numbers,” reports the BBC today, “it recorded some 314,835 new coronavirus cases in the past 24 hours, while deaths rose by 2,104.
With European CE-IVD approval announced March 18, 2021, Xphyto’s lead diagnostic product, Covid-ID Lab, XPhyto continues to enhance its commercial team as it anticipates near-term distribution and sales.
- Lukas Kane
Full Disclosure: Xphyto is an Equity Guru marketing client.


Leave a Reply