Revive Therapeutics (RVV.C) signed a research agreement with the University of California, San Francisco (UCSF) to explore Bucillamine as a potential treatment for severe cases of COVID-19 today, according to a press release.
The research will be done by Dr. John Fahy with the intention of testing the efficacy of Bucillamine in pre-clinical models of COVID-19 and also to design protocols for human testing.
“We are excited to expand the use of Bucillamine as a potential treatment for severe COVID-19 with our research agreement with UCSF and Dr. Fahy as Principal Investigator. Revive is focused on proving Bucillamine’s clinical utility for all forms of COVID-19. Evaluating Bucillamine for severe COVID-19 along with our dedication in completing our ongoing Phase 3 clinical study for mild-to-moderate COVID-19, which has grown from 14 clinical sites to now 26 participating sites in 10 U.S. states, will position Bucillamine as a potential oral treatment option for mild-moderate to severe COVID-19,” said Michael Frank, CEO of Revive.
Revive therapeutics is a life sciences company involved in the research and development of drugs to treat infectious diseases and rare disorders. It’s priority is putting together the requirements to hop through FDA hoops including the acquisition of Orphan Drug, Fast Track, Breakthrough Therapy and Rare Pediatric Disease designations. At present they’ve been focused on exploring Bucillamine, a drug commonly used with indications like rheumatoid arthritis, for use against severe influenza including COVID-19.
Lately, though, they’ve expanded out to include psilocybin-based therapeutics for various diseases and disorders, as well as having recently acquired orphan drug status for CBD for autoimmune hepatitis (liver disease) and also for ischemia and reperfusion injury from organ transplantation.
What have you done for me lately?
Just because someone’s got the PhD beside their name doesn’t necessarily mean they’re any good at what they do. Where are they published? How recently? With whom? These are the questions we should ask when we see someone call themselves ‘Doctor.’ It’s entirely possible that they’re a practicing doctor in a clinic or with their own private practice, and that’s fine and dandy, but it doesn’t necessarily mean they’re going to be any good in a clinical setting. Research skills get rusty like anything else.
Here’s Dr. Fahy’s credentials from the press release:
Dr. John Fahy, MD, MSc is a Professor of Medicine in the Division of Pulmonary and Critical Care Medicine and the Department of Medicine at the University of California, San Francisco and is a director of UCSF’s severe asthma clinic. He also directs the UCSF Airway Clinical Research Center. His research receives funding from the National Institutes of Health for studies of lung disease and for studies of thiol-based drugs to treat mucus pathology in the lung. Dr. Fahy earned his medical degree at the University College Dublin. After internal medicine training in Dublin, he completed fellowship training in pulmonary and critical care medicine at UCSF. He is the Michael S. Stulbarg Endowed Chair in Pulmonary Medicine.
Dr. Fahy’s recent study is called “Thiol-based drugs decrease binding of SARS-CoV-2 spike protein to its receptor and inhibit SARS-CoV-2 cell entry.” It shows that thiol-based drugs, of which Bucillamine is one, decrease the binding of SARS-CoV-2 spike protein to its receptor, and therefore decrease the efficiency of the virus’s ability to infect new people, and could theoretically help stem the spread.
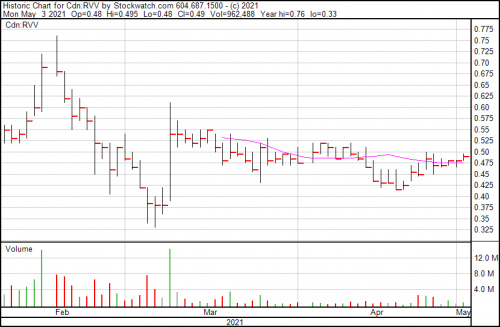
Revive Therapeutics is up a penny today and presently trading at $0.49.
—Joseph Morton
Full disclosure: Revive Therapeutics is an equity.guru marketing client.

Leave a Reply