Last year Big Pharma unleashed 1,500 lobbyists to Washington, DC, who then cut cheques for $177 million to keep drug costs high.
The lobbyists deserve bonuses.
The average U.S. citizen spends $1,200 per year on prescription medicines, while people in other western countries spend an average of $550/year.
Public health data indicates that the gluttony and sloth of Americans is causing them health problems – but even factoring this in – U.S. residents are still getting bilked by drug makers.
Following the reductive ethics of the stock market, high-margins are always desirable.
To get a green light to sell prescription drugs in the U.S, biotech companies need approval from the Federal Drug Administration (FDA). Receiving a “thumbs down” from the FDA can destroy the stock price of a small cap biotech company.
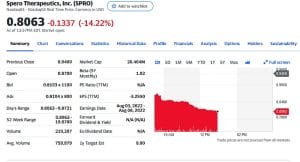
Example: on June 27, 2022, in after-hours trading Spero Therapeutics (SPRO.Q) announced that “the FDA declined to approve its oral antibiotic drug for the treatment of patients with complicated urinary tract infections”.
The health regulator concluded that Spero’s late-stage study testing the drug was insufficient and an additional study would be required. Shares of Spero fell 14% the next day.
Biotech companies receiving “thumbs-up” from the FDA, tend to be favored by investors.
Revive Therapeutics (RVV.C) is one such company.
Revive is researching and developing therapeutics for infectious diseases and rare disorders, and it is prioritizing drug development efforts to take advantage of several regulatory incentives awarded by the FDA such as:
- Orphan Drug
- Fast Track
- Breakthrough Therapy
- Rare Pediatric Disease designations
Being accepted into any of the above categories removes some hurdles and expenses, while creating a smoother pathway to commercialisation.
RVV is exploring the use of Bucillamine for the potential treatment of infectious diseases, with an initial focus on severe influenza and COVID-19.
With its acquisition of Psilocin Pharma Corp, Revive is also advancing the development of Psilocybin-based therapeutics in various diseases and disorders.
Revive’s cannabinoid pharmaceutical portfolio focuses on rare inflammatory diseases and the company was granted FDA orphan drug status designation for the use of Cannabidiol (CBD) to treat autoimmune hepatitis (liver disease) and to treat ischemia and reperfusion injury from organ transplantation.
On June 24, 2022 RVV provided an update on the company’s FDA Phase 3 clinical trial to evaluate the safety and efficacy of Bucillamine, an oral drug with anti-inflammatory and antiviral properties, in patients with mild to moderate COVID-19.
Following RVV receiving positive comments from the FDA in regards to the Company’s request to determine and agree on the Study’s potential new primary efficacy endpoints and the Company’s submission of a Data Access Plan to the FDA, the FDA has accepted the DAP to allow for the unblinding of the pre-dose selection data.
The Company will now proceed to unblind the pre-dose selection data to potentially support the amended Study protocol with the new primary efficacy endpoints.
The proposed new primary efficacy endpoints may include the rate of sustained clinical resolution of symptoms of COVID-19, which addresses the shift in COVID-19 clinical outcome observed over the course of the pandemic, and, therefore, to have more meaningful study endpoints for the FDA to consider for potential Emergency Use Authorization.
The Company believes that with the Omicron variant, including the BA.2 variant, being the dominant strain over the Delta variant, there is an urgent need to treat symptom resolutions in addition to preventing hospitalizations.
The FDA is giving RVV increased flexibility to create meaningful comparative data.
On May 31, 2022, Revive announced that in light of the growing cases of acute hepatitis in children reported by the World Health Organization (WHO), it will advance its drug pipeline for inflammatory liver disorders including Bucillamine in the prevention of ischemia-reperfusion injury during liver transplantation and Cannabidiol for autoimmune hepatitis.
As of May 26, 2022, six hundred and fifty probable cases of acute hepatitis of unknown aetiology in children have been reported to WHO from 33 countries in five WHO Regions between 5 April and 26 May 2022.
At least 38 children have required a liver transplant, nine have died, and 99 cases pending classification, according to a statement from WHO.
A month ago, Trade to Black did an excellent deep-dive interview with the knowledgeable articulate Biomedical Engineer and RVV investor, Arri Morris.
“Most research out there is focused on attacking the virus, and really antagonizing COVID itself,” explained Morris, “This drug Bucillamine is aimed at the person and improving clinical outcomes for patients.”
“The problem with attacking the virus itself, is that this virus is a shapeshifter. We get a new variant pretty regularly now. If you have something aimed at the virus, it’s likely to stop working in the future. If you have something aimed at helping the patient, it’s much more durable, and I think will stand the test of time”.
At the height of the pandemic, RVV was trading at .68.
By March 28, 2022 the stock price had fallen to .18.
Since then, amidst a slew of good news from the FDA, the stock has more than doubled to .43 – with a current market cap of $139 million.
Try Googling “Bucillamine” and you’ll discover that it is a sort of medical Swiss-Army-Knife with an array of utilities including “regulating B-cell function” “thiol antioxidant” and “cisplatin-induced otoxicity”.
Revive Therapeutics is exploring the use of the drug Bucillamine as a potential novel treatment for infectious diseases including influenza and the coronavirus disease (Covid-19).
Investors should keep their eyes on the Phase 3 trial. The current noises from the FDA are positive.
At this point, commercialization of RVV’s products is more than a pipe dream.


Leave a Reply